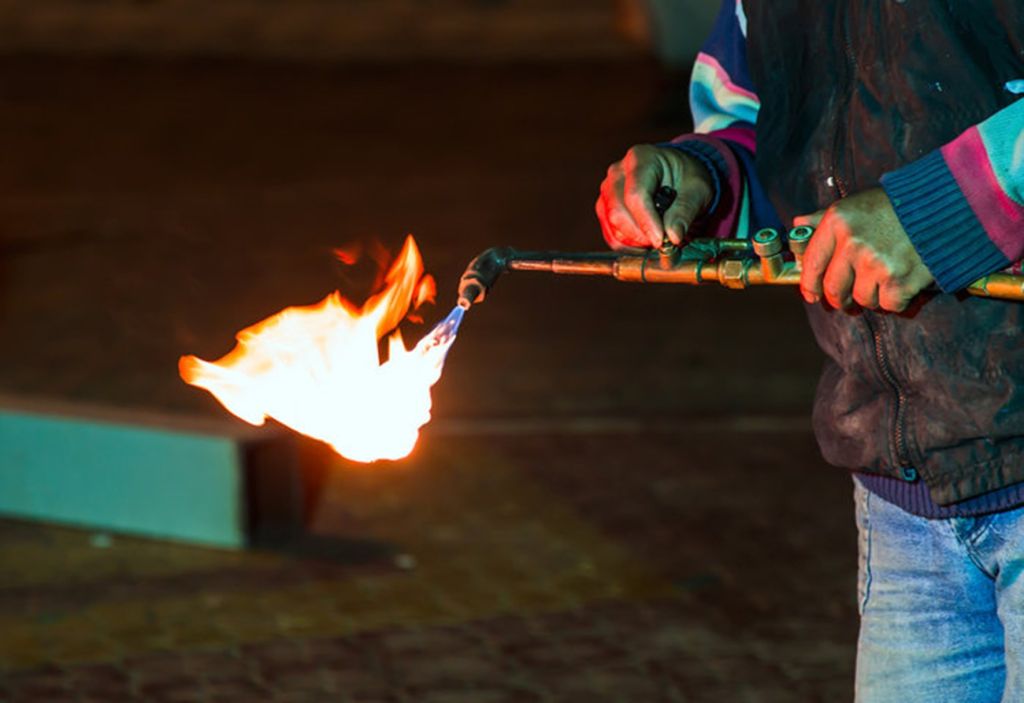
Acetylene is one of the most important compounds in the chemical and manufacturing industry, used in a variety of processes due to its unique properties. In this article, we'll explore what acetylene is, what industries it's used in, the processes it uses, its associated hazards, and the safety measures needed to work with it.
What is Acetylene?
Acetylene, or ethylene, is an unsaturated hydrocarbon with the chemical formula C₂H₂. It is a colorless and highly flammable gas that is in a gaseous state at normal temperature and pressure. It is produced by the reaction of calcium carbonate with hydrochloric acid or by the decomposition of calcium carbide with water.
Industries Using Acetylene
Acetylene is a key raw material in several industries due to its high-energy properties and responsiveness. Among the main industries that employ it are:
- Chemical Industry: Acetylene is used as a raw material for the production of essential chemicals such as vinyl chloride and acrylonitrile.
- Metallurgical Industry: It is used in welding and metal cutting due to its high flame temperature.
- Plastics Industry: It is a precursor for the manufacture of polymers and plastics such as polyacetylene.
- Pharmaceutical Industry: It is used in the synthesis of certain drugs and pharmaceutical compounds.
Processes in which Acetylene is Used
Acetylene has a number of key industrial applications, including:
Welding & Cutting
- Acetylene Welding: Used in combination with oxygen to perform strong and precise welds on metals.
- Acetylene Cutting: Used to cut metals at high temperature.
Chemical Production
- Ethylene Synthesis: Acetylene is converted into ethylene, a building block for the production of various chemicals.
- Acetaldehyde Synthesis: Used in the production of acetic acid and other substances.
Plastics & Polymers
Polyacetylene: It is produced from acetylene and is used in electronic applications and as a conductive material.
Why Is Acetylene So Dangerous?
Acetylene is extremely dangerous due to its characteristics:
- Highly Flammable: Forms explosive mixtures with air and ignites easily.
- Instability: At high pressures, acetylene can break down violently, causing explosions.
- Toxicity: In high concentrations, it can be toxic and asphyxiating.
Safety Measures for Working with Acetylene
Due to the risks associated with acetylene, it is crucial to follow strict safety measures. Here are some key recommendations:
Handling and Storage
- Storage in Proper Cylinders: Use approved acetylene cylinders and keep them upright.
- Proper Ventilation: Make sure workplaces have adequate ventilation to prevent dangerous buildup.
- Avoid Contamination: Do not store acetylene near heat sources or combustible materials.
Working Procedures
- Use of Personal Protective Equipment (PPE): Wear safety glasses, gloves, and fire-resistant clothing.
- Regular Maintenance: Regularly inspect equipment and cylinders for leaks or damage.
- Training: Provides ongoing training on risks and safe practices to all employees.
ACETILEX: The EGA Master solution
Acetylene is a group IIC gas, and therefore non-sparking alloys do not have the capacity to cause its deflagration. However, this type of gas reacts with any alloy that is composed of more than 65% copper, where copper acetylide is generated.
As both the non-sparking alloys Cu-Be and Al-Bron contain more than 80% copper, it makes it unfeasible to use this type of alloy in explosive atmospheres where acetylene is present.
There are other non-sparking alloys other than Cu-Be and Al-Bron, such as brass.
However, despite the fact that brass is composed of 60% copper, its mechanical strength is very low compared to copper-beryllium and bronze aluminum non-sparking alloys, specifically, between 4 and 6 times less mechanical resistance.
This results in a shorter useful life, and a very unprofitable option.
For this reason, at EGA Master we have developed the ACETILEX non-sparking tools, which are Cu-Be and Al-Bron non-sparking alloys whose copper percentage is less than 65%, making them the only safe and profitable non-sparking option in environments with acetylene.
Do you work in environments where acetylene is present? Contact us for advice from our experts.

